Recent work by MDPRC members
Yang, M. et al. (2025). Separate orexigenic hippocampal ensembles shape dietary choice by enhancing contextual memory and motivation.
Yang et al. reveal that the hippocampus (HPC) - previously shown to facilitate food inhibition - also plays a surprising and powerful role in driving nutrient-specific food intake. The authors identified and manipulated sugar- and fat-responsive neuronal populations in the dHPC, to enhance spatial memory for sweet rewards and boost motivation for fatty foods —offering new insight into how our brains can drive overeating and opening the door to potential obesity interventions.
Read the full article here.
Minère, M. et al. (2025). Thalamic opioids from POMC satiety neurons switch on sugar appetite.
The authors uncover a novel mechanism by which hypothalamic POMC neurons, classically associated with satiety signaling, contribute to sugar-specific appetite in the sated state. They show that POMC neurons engage a mu-opioid receptor-mediated inhibitory projection to the paraventricular thalamus, selectively activated during sugar consumption. These findings provide new insight into the neural substrates that enable hedonic feeding despite metabolic sufficiency.
Read the full article here.
Chang, H. et al. (2024). Stress-sensitive neural circuits change the gut microbiome via duodenal glands.
This study identifies a circuit through which stress-sensitive brain regions regulate gut microbiota and host defense, demonstrating that glands in the duodenum mediate vagus nerve-dependent enrichment of Lactobacillus species, a process disrupted by either gland ablation or chronic stress. Activation of this circuit restores microbiome composition and immune protection under stress, establishing a mechanistic link between brain state and microbial homeostasis.
Read the full article here.
Thanarajah, S.E. et al. (2023). Habitual daily intake of a sweet and fatty snack modulates reward processing in humans.
Thanarajah et al. clarify the causal link between obesity and altered dopamine function. They did this through a randomized, controlled study wherein normal-weight adults were exposed to either a high-fat/high-sugar or low-fat/low-sugar snack in addition to their regular diet for 8 weeks. By the end of the study, those exposed to the high-fat/high-sugar intervention showed decreased preference for low-fat foods, increased brain response to food, and associative learning independent of food cues or reward. Controlled for metabolic and weight changes, these findings indicate a direct effect of high-fat, high-sugar foods on neurobehavioral adaptations that may increase the risk for overeating and weight gain
Read the full article here.
Montalban, E. et al. (2023). The addiction-susceptibility TaqIA/Ankyrin repeat and kinase domain containing 1 kinase (ANKK1) controls reward and metabolism through dopamine receptor type 2 (D2R)-expressing neurons.
Homozygous expression of the A1 allele of the TaqA1 polymorphism on the Ankk1 gene correlates with reduced striatal D2R. Montalban et al. reveal the effect of Ankk1 on D2R-expressing striatal neurons and subsequent alterations in learning, impulsivity, and cognitive flexibility endophenotypes. They also uncover a novel link between Ankk1 in D2R-expressing neurons and energy homeostasis and nutrient partitioning.
Read the full manuscript here.
Zhang T., Perkins M. H., Chang H., Han W., de Araujo I.E. (2022). An inter-organ neural circuit for appetite suppression.
Zhang et al. explore the peripheral mechanisms of endogenous GLP-1 action. The authors contribute the hypothesis that intestinal GLP-1 triggers gut-brain neural transmission by recruiting local enteric circuitries. Pubmed Link.
Perszyk E.E. et al. (2021). Fat and Carbohydrate Interact to Potentiate Food Reward in Healthy Weight but Not in Overweight or Obesity.
This research replicates in an American sample prior findings that energy density drives the reinforcing value of food, that energy from fat and carbohydrates can interact to potentiate reward, and that this interaction is strongest in healthy weight, but not overweight individuals. Pubmed Link.
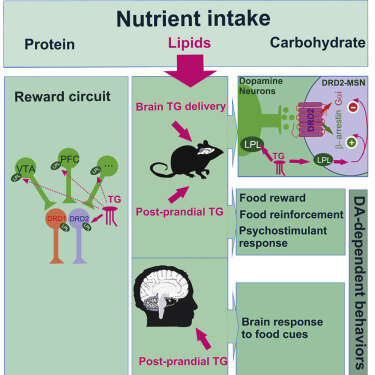
Berland C. et al. (2020). Circulating Triglycerides Gate Dopamine-Associated Behaviors through DRD2-Expressing Neurons.
This paper offers insight into how energy-dense foods affect dopamine transmission in the mesocorticolimbic system, showing how triglycerides modulate this behavior and unveiling a trend among individuals with genetic risk of reduced DRD2 signalling. PubMed Link.
Dalenberg, J.R. et al. (2020). Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans.
This research paper explores the contentious question of whether low-calorie sweeteners increase the prevalence of obesity and related comorbidities, showing that their joint consumption with carbohydrates impairs metabolic response. The findings in this paper challenge the sweet uncoupling hypothesis. PubMed Link
de Araujo, I.E., Schatzker, M., and Small, D.M. (2020). Rethinking Food Reward. Annu. Rev. Psychol. 71.
This review offers a new model for food reward where gut-brain reward pathways bypass cranial taste and aroma sensory receptors and cortical networks that give rise to flavor perception and instead reinforce behaviors independently of the cognitive processes that support overt insights in the nature of our dietary decisions. PubMed Link
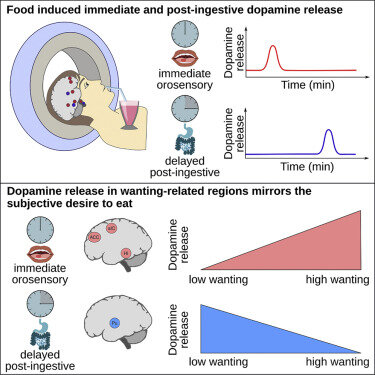
Thanarajah, S.E. et al. (2019). Food Intake Recruits Orosensory and Post-ingestive Dopaminergic Circuits to Affect Eating Desire in Humans.
This original research combines fMRI and PET methods to reveal dopaminergic circuits influencing desire to eat. PubMed Link
Small, D.M., and DiFeliceantonio, A.G. (2019). Processed foods and food reward. Science 363, 346–347.
This paper provides a brief perspective on how processed foods might affect physiology in unanticipated ways that could promote overeating and metabolic dysfunction. PubMed Link
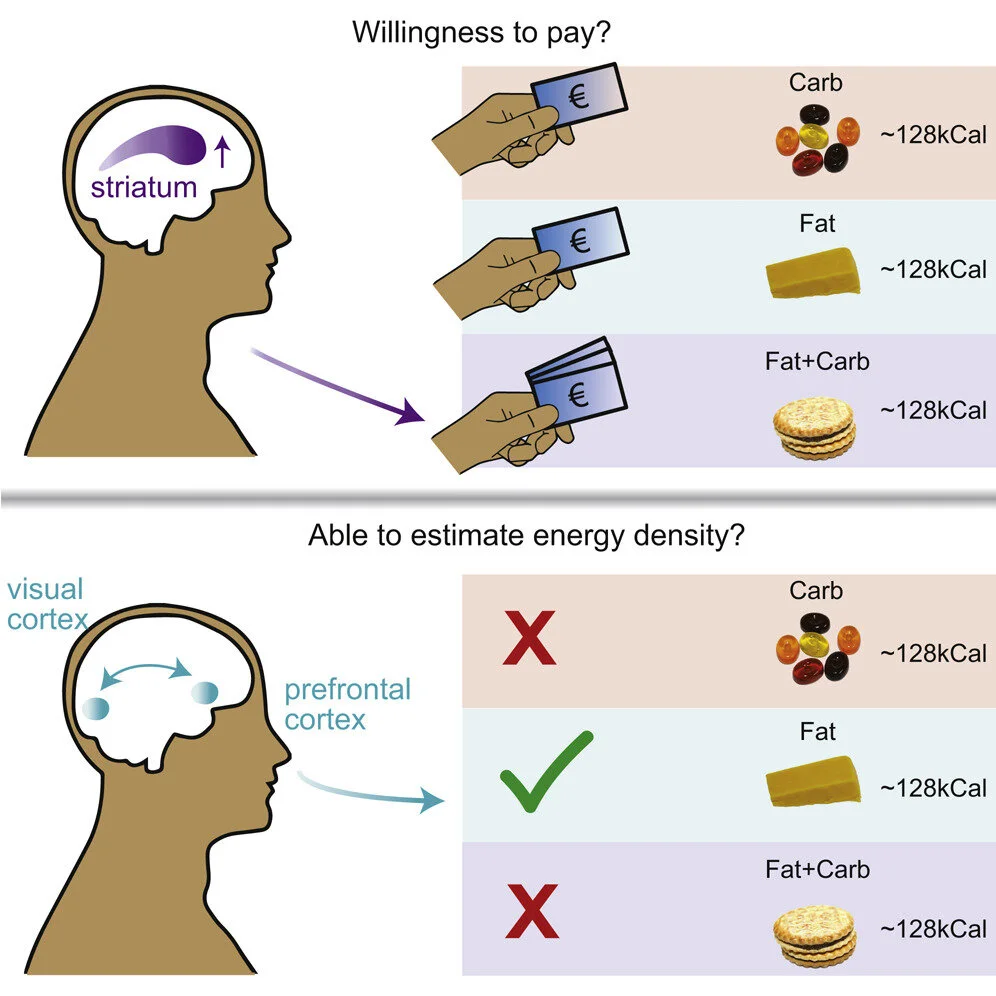
DiFeliceantonio, A.G. et al. (2018). Supra-Additive Effects of Combining Fat and Carbohydrate on Food Reward. Cell Metab. 28, 33-44.
This original research paper shows that foods containing fat and carbohydrate are more reinforcing that equally caloric foods containing primarily fat or carbohydrate. This effect is independent of liking and is reflected by supra-additive responses in brain reward regions. This may be one mechanism driving overconsumption of energy dense processed foods. PubMed Link
Han, W. et al. (2018). A Neural Circuit for Gut-Induced Reward. Cell 175, 887–888.
This landmark original research paper maps a gut-to-brain neural circuit linking nutrient sensing in the gut to central reward circuits. It provides a mechanism by which the peripheral signals directly influence central circuits to regulate food reward. PubMed Link